Immunoglobins, like most other small structures in the body, are polypeptide chains (proteins). Proteins, and therefore, immunoglobins, are comprised of amino acids. Each immunoglobin has 4 major sections: 2 heavy chains and 2 light chains, which can be abbreviated as H and L respectively. The heavy chains have approximately 400 peptides (amino acids) each and the light chains have approximately 200 chains each (Marieb). The chains have loops, which can be seen in the figure below. These loops are caused by disulphide bonds (sulfur-sulfur covalent bonds) between different parts of the chain. They occur after about every 110 peptides (Marieb). The general shape of antibodies is a Y; however, different variants of antibodies may be comprised of several Y structures linked together. The region where the chain bends is called the hinge region.
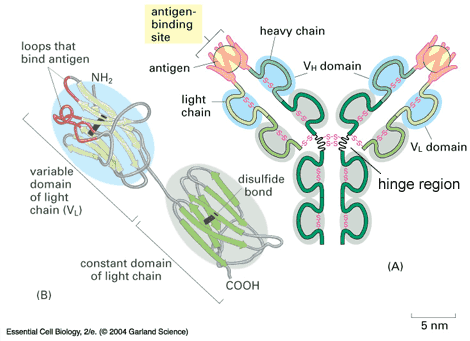
The crystallizable fragment is the 'stalk' of the Y. This portion of the immunoglobin determines what will happen to the antigen when the immunoglobin bonds to it, and how it is classified. It acts as a receptor. Its function in this capacity will be discussed in greater depth in the immunoglobin-antigen bonding section.
The antigen binding fragment is the area of the immunoglobin, which determines what antigens it can bond with. It is comprised of 2 subsections. The Hypervariable/Complementary Determining Region (HV/CDR) is the specific region, which determines what antigens the immunoglobin can bond with. It varies greatly between different antibodies to provide greater immune diversity. There are 3 of these regions (http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html). The 4 Framework Regions (FR) provide structural support so that the shape of the binding site and antigen can be complimentary, facilitating bonding (http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html). They are not directly involved in the bonding process (http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html). This region does not vary as much between antibodies as the CDR.
In humans, there are 5 major types of antibodies, which come in 3 major forms: monomers, dimers, and pentamers. Monomers are the traditional Y shaped antibodies. Dimers are to Y antibodies, which have their crystallizable fragments joined. Pentamers are Y antibodies, which have their crystallizable fragments joined in a pentagon formation. The type of antibody is determined by its crystallizable fragment. All antibody type names begin with Ig, which stands for Immunoglobin. Some types of antibodies can come in multiple forms under different circumstances.